*CARACAL
CARACAL stands for the Competent Authorities for Registration, Evaluation, Authorisation and restriction of Chemicals (REACH) and Classification, Labelling and Packaging (CLP). In the regular CARACAL meetings, expert groups, stakeholders and the REACH authorities come together to discuss current topics concerning the REACH Regulation (EC) No 1907/2006 and the CLP Regulation (EC) No 1272/2008. One of the hot topics at present time is the REACH Revision. The presented updates are a first version of the proposed changes for REACH and are still under discussion.
(Q)SAR and Read Across Analyses in Regulatory Assessment of Pesticide Metabolites and Impurities in the Light of Current Insights and Demands
Authors: Svenja Termeer de Amanqui, Volker Harder

Consumers can be exposed to pesticide residues potentially containing active substances (a.s.) and concomitantly to impurities and residue metabolites. As these substances may have properties of concern for human health, they need to be evaluated. In contrast to the comprehensive toxicological data set of an a.s., toxicological information on metabolites and impurities is generally scarce or non-existent. Based on legislation there is the possibility to apply New Approach Methodologies to assess the toxicological relevance of a.s. metabolites and impurities. (Quantitative) structure activity relationship ((Q)SAR) and read across (RA) analyses enable rapid and feasible hazard assessments.
Metabolites are evaluated according to the EFSA PPR Guidance (2016) using the EFSA templates (2020) for assessing (Q)SAR reports and for summarizing and integrating the evidence. The forthcoming OECD Guidance on residue definition (2023) is expected to further demand additional RA/grouping. To assess relevance of impurities the Guidance on assessment of equivalence (2012) is followed.
Reflecting all recommendations, robust in silico predictions are generated for genotoxicity and other toxicity endpoints by complementary rule- and statistics-based (Q)SAR software tools. The reliability of the predictions is evaluated based on the prediction’s evidence. Knowledge of the chemistry/biology regarding the endpoint is needed for overall evaluation in a weight of evidence approach. The OECD QSAR Toolbox is additionally used for toxicity screening, assessment of organic functional groups, calculation of physico-chemical properties and similarity to facilitate grouping, selection of appropriate analogues and data gap filling.
Based on all information, a case is established with a rationale, and if needed with a testing proposal, to support the conclusion for dietary risk assessment of metabolites or for the evaluation of the relevance of impurities. State-of-the-art reports describe strategy, methods followed, and results obtained.
In summary, in silico methods are an efficient and accepted tool in the assessment of the toxicological relevance of pesticide metabolites and impurities.
References:
EFSA PPR Panel, 2016, Guidance on the establishment of the residue definition for dietary risk assessment, EFSA Journal 2016;14(12):4549, 129 pp. doi:10.2903/j.efsa.2016.4549
EFSA, 2020, Technical report on the outcome of the pesticides peer review meeting on general recurring issues in mammalian toxicology, EFSA supporting publication 2020:EN-1837. 26 pp. doi:10.2903/sp.efsa.2020.EN-1837
EC, 2012, Guidance document on the assessment of the equivalence of technical materials of substances regulated under regulation (EC) No 1107/2009, SANCO/10597/2003 –rev. 10.1
Key words: impurities, in silico, metabolites, New Approach Methodologies, pesticide active substance.
REGULATORY NEWS
Efficacy and product performance – subject of change
Date: 15 December 2020
With respect to the often time- and cost consuming efficacy data requirements for plant protection product authorisations, publication of EPPO standard PP1/296(1) on the “Principles of efficacy evaluation for low-risk plant protection products” in 2017 was one of the very few interesting regulatory innovations in the last decades. But now, EU’s Green Deal and the related Farm to Fork (F2F) strategy have the potential for significant changes in regards to efficacy and product performance.
Devised as “roadmap for making the EU's economy sustainable by turning climate and environmental challenges into opportunities across all policy areas” (EU COM 11 December 2019), EU’s Green Deal has an outstanding importance for agriculture. Considering the nature and objectives of the F2F concept there are many opportunities, especially in regards to synergies between the regulatory approval and authorisation process on the one hand as well as sales and marketing of plant protection products on the other hand. As a direct link between these issues, efficacy and performance of plant protection products will play a key role and gain in importance due to the requirements listed in the F2F strategy. One example in this regard is the mandatory implementation of the Leaf Wall Area (LWA) concept in the European Central Zone which aims at reducing unnecessary pesticide outputs in high growing crops. Further opportunities but also challenges arise from the stricter implementation of integrated production (IP) and integrated pest management (IPM) principles, considering the whole cornucopia of possibilities to achieve a sustainable agriculture. This comprises classical topics such as crop rotation, mechanical, physical, biological and chemical measures, supplemented by precision and digital farming, etc., and of course it includes also new technical and scientific developments on a broad range of fields, such as development of new formulation and related application types to reduce dose rates to be applied or innovative active substance categories such as elicitors or plant activators.
While compatibility with IPM was in the past only worth a sub head in PPP dossiers, the subject will gain more and more attention, especially for chemical pesticides. Where up to now the simple proof of effectiveness was enough as “raison d'être” for a chemical pesticide, proven compatibility and contribution to an IPM strategy may in future make the difference, whether authorities(re-)authorize or (re-)register a chemical product or not, and – with regards to sales – whether farmers perpetuate to consider a specific chemical PPP in their application schedule or not.
More than ever, the mandatory official assessment of product performance and efficacy in the EU will guarantee farmers the product effectiveness, safety and economic benefits due to its application. As such, product performance and efficacy is the direct linkage between agricultural practise, marketing and sales of plant protection products, biostimulants, fertilisers and adjuvants and their registration – for which F2F can be a challenge but also an opportunity.
For more information on SCCs independent handling of efficacy and product performance testing and assessment please visit our website as well as our regulatory guidance on “efficacy in a nutshell”.

SCC Newsletter Vol. 20, No. 2, December 2020
REGULATORY NEWS
Efficacy testing – new EPPO standard for plant defence inducers (PDIs)
Date: 15 December 2020
EPPO has published a new standard (PP1/319 (1) on the general principles for efficacy evaluation of plant protection products with a mode of action as plant defence inducer (PDI). The standard does not cover plant biostimulants as defined by the EU Fertiliser Regulation 2019/1009 laying down the rules on the making available on the market of EU fertilising products. The new European Fertilizer Regulation including biostimulants entered into force in July 2019 and is scheduled to fully apply from July 2022 onwards. Thus, PDI substances are considered to be plant protection products and Regulation 1107/2009 applies.
The standard defines PDIs as follows: “Plant defence inducers (PDIs, also known as plant defence elicitors) include any substance (products of synthetic or natural origin or micro-organisms) which, when applied to a plant, can induce a state of local and/or systemic resistance against biotic stress. PDIs are perceived by plants as a signal of danger and do not target the pest directly. They act to develop or implement different defence mechanisms, leading to increased plant resistance to pests”.
Demonstration of efficacy for PDIs should be based on:
- Preliminary studies especially targeting the Mode of Action (MoA)
The Standard highlights the importance to evaluate and demonstrate the specific Mode, or Modes, of Action by preliminary studies. Based on the product characteristics the Standard suggest several methods such as transcriptional analyses, protein analyses or metabolic analyses to describe the activating or priming defence mechanisms triggered by the specific product.
In addition, in the scope of preliminary studies the standard requires demonstration of the absence of significant direct effects on the pest species to be controlled. For such tests in vitro testing on solid or liquid media is required.
- Efficacy trials demonstrating the level of efficacy of the PDI
The Standard does not replace any of the already available, relevant general EPPO standards but explicitly refers to standards such as PP 1/181, PP 1/226, PP 1/152 or PP 1/225 for general layout and design of efficacy trials. This is also due for low risk products for which the Standard refers to EPPO Standard PP 1/296 on the principles of efficacy evaluation for low risk plant protection products, in particular in regards to information on the required number of trials. Similar in the case of three-dimensional crops for which EPPO Standard PP 1/239 on dose expression for plant protection products has to be used.
Due to the Mode(s) of Action of PDIs, the Standard highlights the importance of application timing and application frequency, the possible delay in plant response, the physiological state of the crop and the persistence of action of the product and its potential cumulative effect to be considered in study design and layout.
Special considerations are required by the Standard in case of tank mixtures or if the product is to be used as a component of an Integrated Pest Management (IPM) strategy. In case specific label claims are added in these regards, evidence from specific efficacy trials have to be provided and the level of efficacy of the PDI as a component in an IPM programme or tank mixture application scheme has to be shown. This is especially due in case of an intended reduction in the dosage or the number of applications of conventional products.
For more information on related topics (such as IPM, low risk product efficacy and product performance, etc.) please refer to our website:
REGULATORY NEWS
Adjuvant registration
Date: 15 December 2020
Adjuvants are products such as wetting agents, stickers and anti-foaming agents which are added and mixed with a plant protection product (PPP) to improve the efficacy of a PPP, to allow better adherence of products for seed treatment or to prevent excessive foam formation.
Before an adjuvant can be placed on the market an authorisation is required. In general, adjuvants fall within the scope of the Regulation 1107/2009 (the Plant Protection Products Regulation. Detailed rules for the authorisation of adjuvants, including data requirements, notification, evaluation, assessment, and decision making procedures are foreseen in Art. 58(2) of the Regulation but still have not been finalised on EU level. For example, Annex III of Reg. (EC) No 1107/2009 includes a list of co-formulants which are not accepted for inclusion in plant protection products, including adjuvants, as referred to in Article 27. Currently this list does not include any entries. Due to the lack of harmonised rules at EU level, adjuvant registration is currently handled nationally on Member State level according to national plant protection laws.
Data requirements and procedures in the Member States differ significantly. In Austria for example no registration procedure for adjuvants exists. The placing on the market of an adjuvant and its correct classification and labelling is solely in the responsibility of the manufacturer. In other Member States such as Germany or the Netherlands a notification/registration procedure exists and information on function, composition, intended use and area of use of the adjuvant needs to be provided as well as a draft label and safety data sheets of the product and its co-formulants. In other countries as for example Belgium or Hungary data requirements are much stricter and already more or less mirror the data requirements for PPP registration according to Regulation 1107/2009 and require data from all sections in dRR-format. However, especially for biological products, data requirements often can be addressed by scientific justifications and literature in spite of studies.
SCC GmbH can provide scientific and regulatory support for national adjuvant registration, starting with a feasibility check or a data gap analysis for your product to develop a registration strategy following up with preparation of final application documents, submission to authority and defence of the application.
REGULATORY NEWS
Metabolites of Microbial Biocontrol Agents (MBCAs) – Guidance on risk assessment published
Date: 15 December 2020
For more than 2 decades the definition, handling, and risk assessment of metabolites of MBCAs – also referred as ’relevant metabolites’ or ‘secondary metabolites’ – is a very controversial subject. Core issues are the unsuited, non-factual definitions and the resulting non-fitting data requirements for microbial metabolites (for more information on this topic please refer for example to Scheepmaker et al. 2019 or Huber et al. 2020 (SCC Current News of 26 August 2020).
Now, European Commission published its guidance working document on the risk assessment of metabolites produced by microorganisms used as plant protection active substances (SANCO/2020/12258). This guidance document ‘addresses metabolites present in the active substance and the plant protection product and also those produced by the microorganism after application (in situ production)’.
Fortunately, for the first time the Commission working document acknowledges that ‘chemical and microbial metabolites are equivalent in name only. Therefore, data requirements concerning metabolites of chemical plant protection products would not be applicable to microbial plant protection products’. Unfortunately, the procedure outlined for risk assessment in the guidance document is based on a strong regulatory but still less scientifically oriented approach. Major aim is to determine whether the microorganism is producing a metabolite of concern, defined as ‘a metabolite produced by the strain under assessment, with known toxicity or antimicrobial activity for which an acceptable risk shall be demonstrated via a quantitative risk assessment’. A stepwise procedure is foreseen:
- Stage 1: Determining the assessment type
- Stage 2: Collecting a basic set of information on metabolites, resulting in a list of metabolites of potential concern
- Stage 3: Determining which of the identified metabolites are of concern, resulting in a list of metabolites of concern
- Stage 4: The risk assessment for metabolites of concern.
On the one hand, the guidance fortunately already includes some scientifically based justifications for waiving of respective risk assessments as for example in the case of viruses for which no further assessment is needed as ‘according to the current scientific knowledge no indication exists that viruses produce metabolites of potential concern’. On the other hand there are several points on which the guidance is less helpful. For example, the natural occurrence and exposure is only partially considered and besides genome sequence data, i.e. proof of absence of genes involved in biosynthesis of a metabolite is the only criterion to exclude a respective stepwise risk assessment. Partially this is attributed to a lack of available scientific data and thus the guidance highlights – or admits - that ‘the present guidance document may be further developed to comply with evolution of science and increasing experience of the EU risk assessors and managers. Firstly, knowledge of microorganisms and microbial metabolites is expected to develop, resulting in the need to reflect such evolution in this document. New scientific and technical approaches supporting the risk assessment of metabolites would need to be incorporated. Moreover, the actual use of microbial plant protection products, and the number of applications concerning microbial active substances are increasing the experience of the EU risk assessors and managers which may trigger a revision of the guidance document’.
The present working document has been finalised in the Standing Committee on Plants, Animals, Food and Feed on 23/10/2020. It will apply to applications submitted from 01/11/2021 onwards.
References
Scheepmaker, J.W.A.,Busschers, M.,Sundh, I., Eilenberg, J. & T.M. Butt (2019): Sense and non-sense of the secondary metabolites data requirements in the EU for beneficial microbial control agents.- Biological Control 136: 1-10.
Huber, L., Lorenz, C. & H. Strasser (2020): Data requirements for biopesticides in EU – problems and needs.- Agrow IHS Markit, Biologicals 2020: 32-34.
REGULATORY NEWS
Update on K-BPR from the Korean government: 5-year vision
Date: 15 December 2020
On November 20th, the Korean Environment of Ministry (MoE) held a public hearing on their plans to develop the Consumer Chemical Products and Biocides Safety Act, better known as K-BPR, in the next five years. SCC attended this event and listed the positive upcoming changes and current limitations of K-BPR.
The products in scope of K-BPR are different as compared to EU BPR. Any chemical product used in the office, home or multi-use facilities is subject to K-BPR. The products in scope can be divided into two groups:
- Chemical products requiring notification, such as detergents, stain removers, bleaching agents, softeners, polishing agents etc.,
- Biocidal products which require approval
Before importing into or manufacturing these products in Korea, importers or manufactures should notify or get approval for their products. In total 80,000 chemical products were notified and approved by August 2020. 74% of these products were air fresheners, candles, detergents and deodorants. Meanwhile, 743 active biocidal substances were notified and are in the approval process.
At the time of the adoption of K-BPR, the Korean government was under significant pressure from the general public to adopt a regulation to prevent further accidents from using chemical products incorrectly. To meet the demand from the general public for swift action, the Korean government used the EU BPR as a template to create its own K-BPR. The swift adoption of K-BPR has led to some inconsistencies and gaps, which the Korean government envisages to address in the coming 5 years.
1. Hazard assessment tool for mixtures
The Korean government is planning to develop a hazard assessment tool for mixtures. Without a K-BPR risk assessment tool today, it is recommended to apply the same tools as used for EU BPR, such as EUSES (European Union System for the Evaluation of Substances). Whether the new, still to be developed, K-BPR assessment tool will be good enough to replace the existing international recognized tools remains to be seen.
2. Acceptance of prediction data
At the moment it is not known how the Korean government will evaluate non-testing data. What is known, so far, is that the government is planning to accept predicting toxicity data, such a SAR and read-across. This will be helpful when using existing EU data for K-BPR dossier preparation.
3. Extension of the chemical products in scope
The range of chemical products subject to K-BPR will be extended from 39 to 50 product types. This means that more chemical products, currently not subject to K-BPR, will be placed in the scope of - the regulation in the coming few years. It is recommended to closely monitor regulatory developments to prevent future surprises.
4. Regulation of microplastics
In line with the developments in the EU, control of microplastics in chemical products will be initiated in the coming five years. The scope and details of the regulation will be decided on the basis of the final EU regulation on microplastics, being currently developed by ECHA. It is expected that the Korean government will apply a similar restriction on microplastics.
5. Using evaluation data from EU and US
In September 2020, the Korean government announced they were accepting applications for a simplified approval for only a few weeks. The simplified approval solely applied to active substances already approved in the EU or US. The Korean government is going to use the information from the simplified approval to screen the remaining active substances on safety. This can mean that the Korean government is going to rely on information from the EU and US for the evaluation of active substances. It is also expected the Korean government is going to focus on active substances which have not been evaluated before in any other jurisdiction.
6. No extensions of existing grace periods for approvals
It has been almost two years since K-BPR was adopted and enforced, yet, there is still no proper technical guidance for preparing or evaluating the dossiers. Korean companies are complaining on the difficulties to prepare the approval dossier without available guidance. However, the Korean government drew a clear line and stated that there will be no extensions of the grace periods.
7. Public database on the evaluation progress
The Korean government is preparing to establish a public accessible database which would allow downstream users to verify the evaluation progress of active substance approvals. In the EU, this information is only shared with the applicants.
8. Active enforcement of K-BPR to verify the safety of chemical products
Due to COVID-19, there is an increasing variety of new biocidal products and treated articles on the Korean market. Therefore, the Korean government is aiming to actively enforce K-BPR and verify the safety and compliance of around 300 chemical products every year. There will be three different ways the Korean government will monitor and enforce K-BPR for the 300 chemical products targeted:
- Purchasing chemical products and verifying compliance with K-BPR through a safety assessment.
- Active enforcement in the market via labelling verifications.
- Reward system encouraging the public to notify chemical products with incorrect or insufficient labelling information.
With the general public taking an active part in the K-BPR enforcement, it is crucial for the companies to keep up-to-date with and implement latest regulation changes applicable to their products on the Korean market.
9. Establishment of a “chemical product evaluation centre”
At the moment there is no independent governmental agency regulating chemicals in Korea, such as ECHA in the EU. The Ministry of the Environment is responsible for the coordination of all activities related to the chemical regulations in place. However, the Korean government is planning to establish an independent governmental agency for evaluating chemical products. This will improve the capacity of the Korean government for processing active substance and biocidal product dossiers.
REGULATORY NEWS
Manual Completeness Check of CSRs by ECHA
Date: 15 December 2020
ECHA announced to extend the Manual Completeness Check to chemical safety reports. The technical completeness of the dossier can be checked by registrants using the validation assistant available in IUCLID. In addition, however, there are further issues not covered by the validation assistant but checked manually by ECHA. The manual verification will contain the following items:
- In case a substance is classified as hazardous or includes PBT properties an exposure assessment and risk characterisation must be included in the CSR
- Exposure scenarios (ES) in the CSR must correspond to all uses reported in the IUCLID dossier
- ES must contain contributing scenarios that correspond to contributing activities reported in the use description (described by the assigned process categories and environmental release categories)
- Required elements of the contributing scenarios are conditions of use, exposure estimates for all relevant routes /compartments and RCRs
If one of the above points is not included in the CSR, a relevant justification needs to be provided. If a relevant justification is not found, the CSR will be regarded as incomplete. As per Article 20(2), quality or adequacy of justifications will not be checked by ECHA.
Further information is available on the ECHA homepage. Although the completeness check of the CSRs has been postponed by ECHA for an indefinite period, it is strongly recommended to thoroughly check the IUCLID dossier for technical completeness by the validation assistant as well as the IUCLID dossier and CSR for compliance before submission to ECHA.
REGULATORY NEWS
EU's new chemicals strategy: A watershed moment
Date: 15 December 2020
With the publication of the Chemicals Strategy for Sustainability on October 14th, the European Commission sets out their new long-term vision for the EU`s chemical policy: a toxic-free environment. The strategy outlines a pathway how these goals can be achieved. This article focuses on the most important actions to be completed by the European Commission in the next years and which might have a big impact on your daily regulatory compliance.
Reducing exposure towards the most harmful chemicals shall be reached by restricting chemicals in consumer products that cause cancers, gene mutations, affect the reproductive or the endocrine system, or are persistent and bioaccumulative. While restriction is only a transitional measure the “generic approach to risk assessment” will become the default option to ensure that consumers, vulnerable groups and the natural environment are most consistently protected. This approach is intended to be extended to further harmful chemicals including those affecting the immune, neurological or respiratory systems and chemicals toxic to a specific organ. Substances exhibiting such properties will be grouped instead of regulating them one-by-one. The first tangible impacts of the strategy can already be found in several updates of regulations and related guidance documents focussing more strongly on the identification of genotoxic substances, e.g. food contact materials and food additives.
Another big topic to be prioritised in 2021 will be the identification of endocrine disrupting chemicals (EDCs) based on the definition of the WHO, and to ensure that they are banned in consumer products as soon as they are identified, allowing their use only where it is proven to be essential for society. Since the EU regulatory system on EDCs is overall fragmented and limited, it needs to be consolidated and simplified across all relevant legislations. Moreover, screening and test methods must be further developed and established in order to generate information on EDCs. Updates of the REACH requirements to allow identification of EDCs are already underway. By taking an international leadership role the European Commission will further promote the introduction of new criteria and hazard classes in UN GHS, i.e. for EDCs but also for terrestrial toxicity and persistency and mobility.
Particular attention will be paid on the protection of the natural environment in general and the use of PFAS in particular, which are to be phased out in the EU unless they are proven essential for society.
People and other living organisms are daily exposed to a wide mix of chemicals originating from various sources. To adequately address the combination effect of chemical mixtures, the Commission will assess how to best introduce ‘mixture assessment factors’ in REACH and introduce or reinforce provisions to take account of the combination effects in other relevant legislation, e.g. food additives and food contact materials, toys and cosmetics.
Industry will face increasing data requirements under REACH, e.g. registration of polymers of concern, assessment of the overall environmental footprint, identification of substances with critical properties including effects on the nervous and immune system as well as carcinogenic substances irrespective of the volume. At the same time a ‘zero tolerance’ approach to non-compliance will be pursued by the Commission and measures to strengthen the enforcement of chemicals legislation could be put in place.
Taken together, new chemicals and materials must be inherently safe and sustainable, from production to end of life, while new production processes and technologies must be deployed to allow the chemical industry’s transition to climate neutrality. The European chemicals strategy for sustainability will affect not just companies that already have a footprint in the bloc of 27 countries, but also those that sell or are looking to sell their products on the EU market. If a company wants to sell something in Europe, it will have to follow the requirements that flow from the new EU strategy, thereby being a big market driver.
REGULATORY NEWS
Date: 15 December 2020
Dear Subscribers,
Welcome to the winter edition of the SCC Newsletter for 2020.
These are extraordinary, challenging times for all of us. So far, we are grateful at SCC we can continue to provide support and advice to all our clients, irrespective of whether we work in the office or from home. Well-developed communication equipment and a restrictive and user-friendly hygiene concept in the company enable us to serve you as you are accustomed from us.
In this issue, you will find features on agrochemical topics (such as efficacy and adjuvant registration), biocides (K-BPR), chemical regulations (e.g. registration updates under REACH) and regulatory science (FOCUS degradation kinetics guidance).
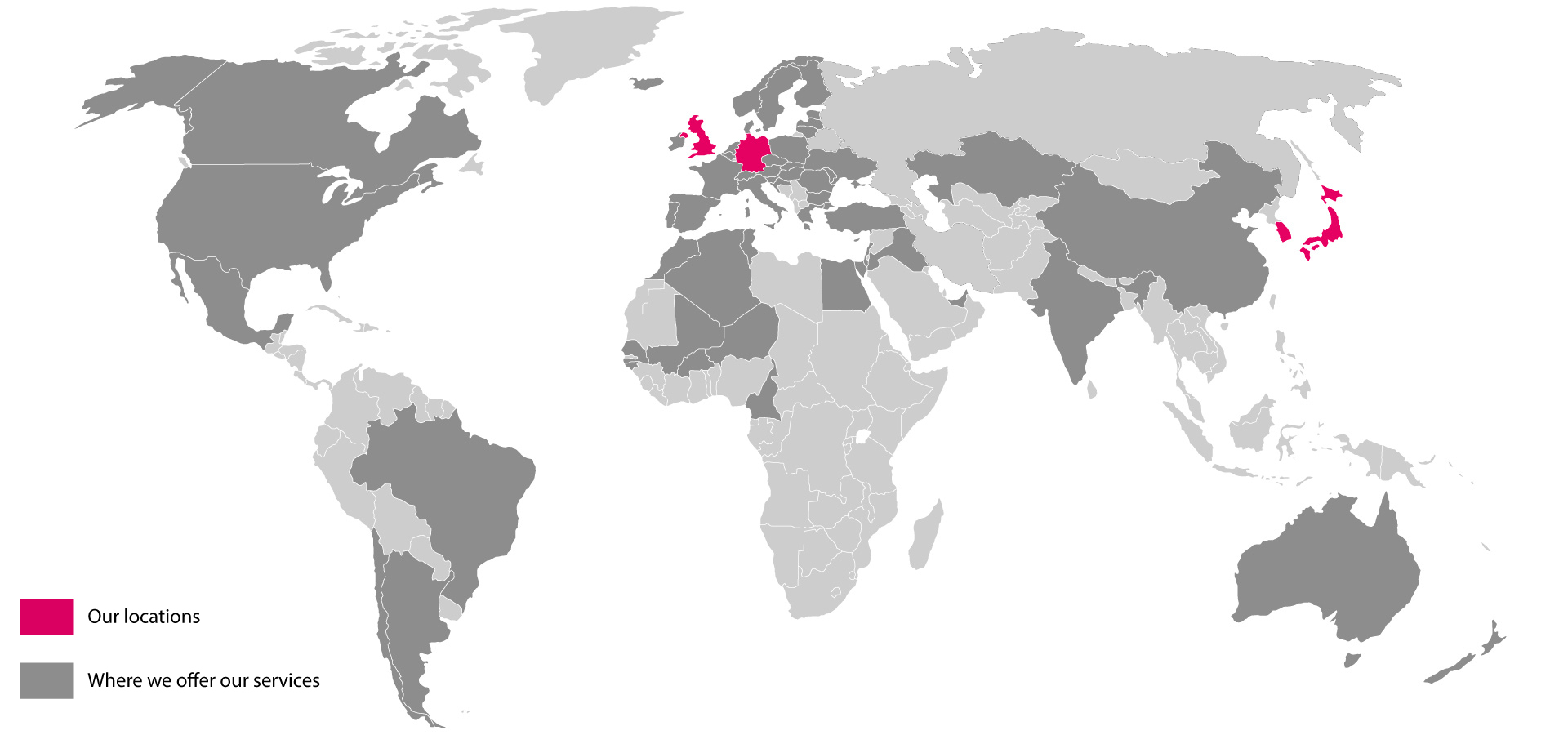
As you might have noticed on our website, we have optimised and enlarged our business services (EU services, International services) and provide regulatory guidance for agrochemicals & biorationals, efficacy and chemicals. We also founded SCC UK in July 2020 to guarantee flexible and unremitting support on short notice, regardless of the Brexit outcome. While the transition period is coming to an end, there is still no substantial progress in negotiations for a trade deal with the most crucial question remaining unclear: Deal or no deal?
In the fast-moving world of regulation, SCC is committed to keeping its customers on course for success. We provide high-quality consulting services for your scientific and regulatory needs. Our expertise extends to exposure modelling and risk assessment and covers a broad range of areas, such as agrochemicals and bio-pesticides, biocides, chemicals, cosmetics, consumer products, feed and food additives, food contact materials, medical devices, GLP archiving solutions, and task force management.
We would love to hear what you think about the SCC newsletter, so please do not hesitate to share your feedback and comments with us. Simply send an email to This email address is being protected from spambots. You need JavaScript enabled to view it..
Finally, all of us here at SCC would like to wish you a joyful festive period despite the COVID-19 situation worldwide. Stay healthy and safe!

Dr Friedbert Pistel